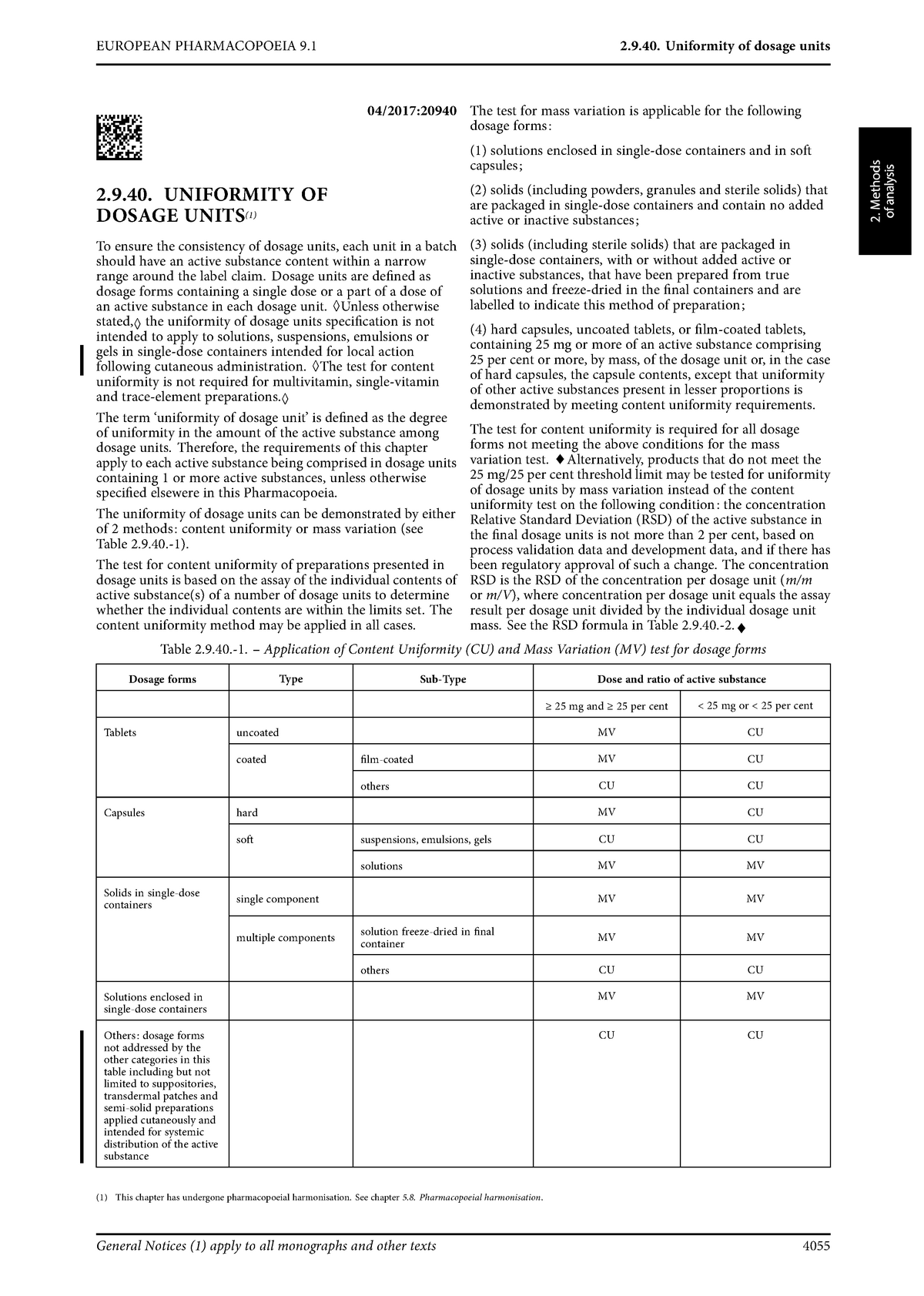
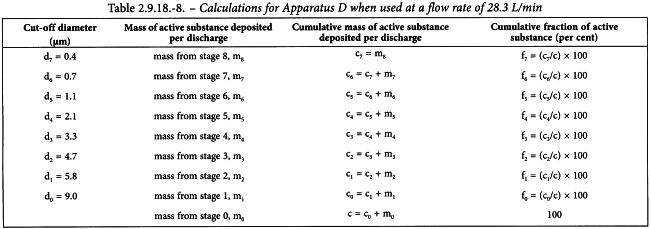
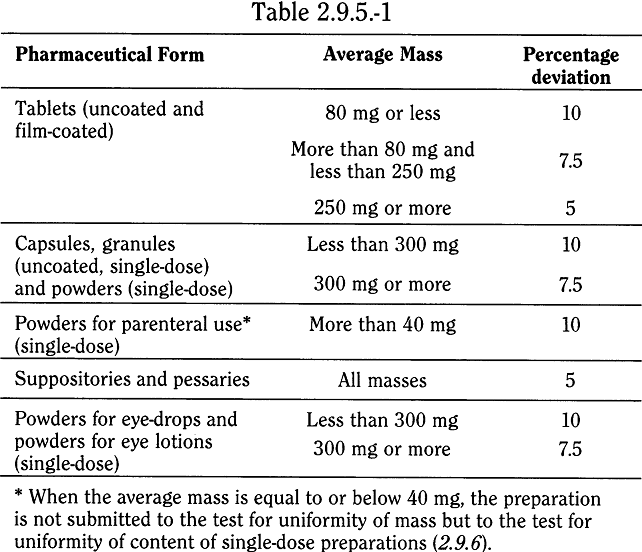
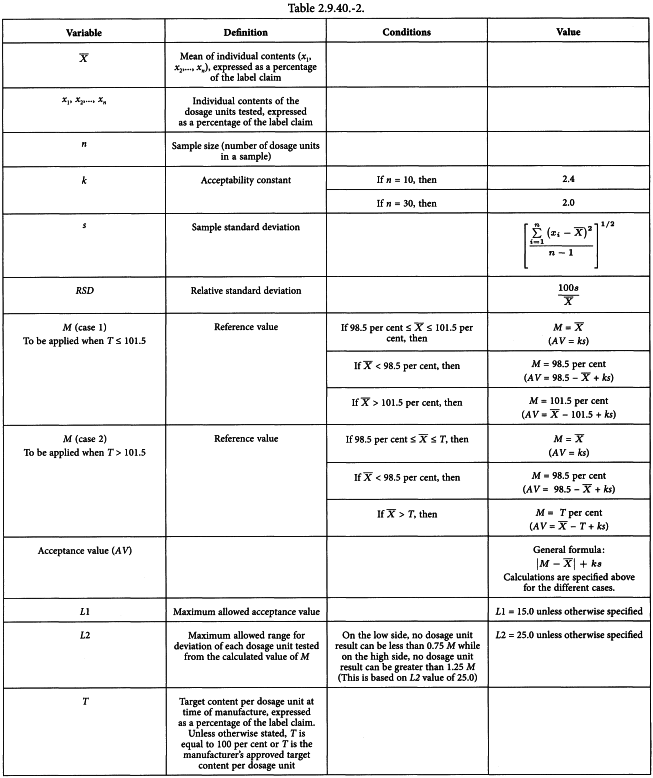
Raman spectroscopy as a complementary tool to assess the content uniformity of dosage units in break-scored warfarin tablets - ScienceDirect

Quality control evaluation of paediatric chocolate-based dosage forms: 3D printing vs mold-casting method - ScienceDirect

Molecules | Free Full-Text | D-Sorbitol Physical Properties Effects on Filaments Used by 3D Printing Process for Personalized Medicine

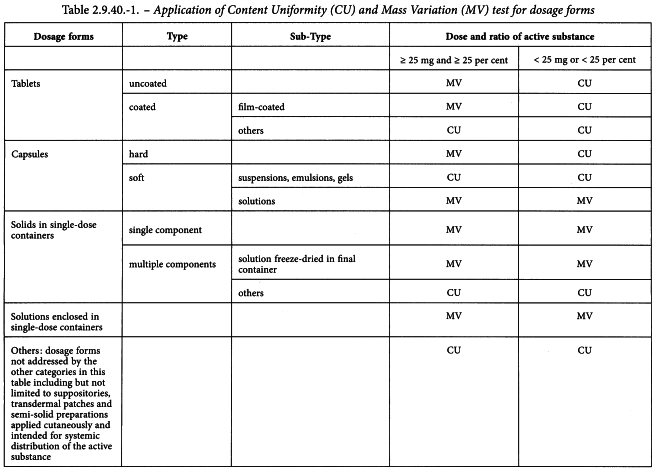
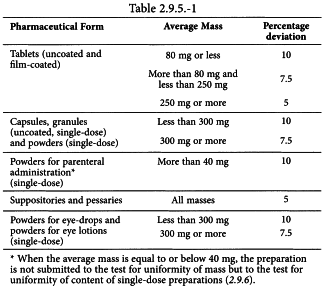
PDF) Mass uniformity: Influence of operational compression conditions of breakability of scored tablets as part of manufacturing robustness evaluation

Evaluation of real‐time data obtained from gravimetric preparation of antineoplastic agents shows medication errors with possible critical therapeutic impact: Results of a large‐scale, multicentre, multinational, retrospective study - Terkola - 2017 -

Pharmaceutics | Free Full-Text | Hot-Melt Extrusion Process Fluctuations and Their Impact on Critical Quality Attributes of Filaments and 3D-Printed Dosage Forms

Physical stability of moisture‐sensitive tablets stored in a canister or as a unit‐dose - Bjerknes - 2017 - Journal of Pharmacy Practice and Research - Wiley Online Library
Pharmeuropa 33.1 just released: don't miss this opportunity to provide your comments - European Directorate for the Quality of Medicines & HealthCare
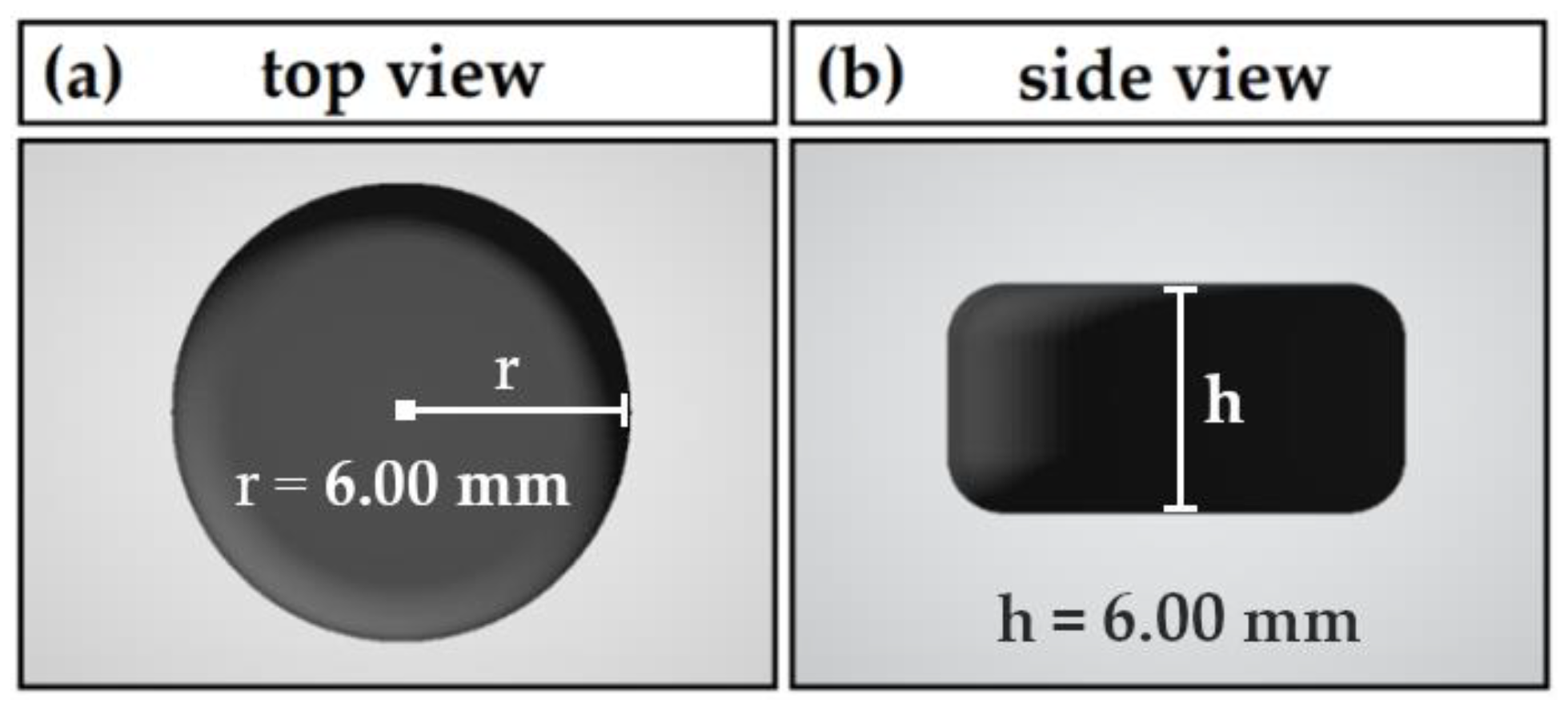
Pharmaceutics | Free Full-Text | Novel Approach to Pharmaceutical 3D-Printing Omitting the Need for Filament—Investigation of Materials, Process, and Product Characteristics